Lessons I Learned From Tips About How To Lower The Freezing Point Of Water

You could perhaps do a project on whether certain types of.
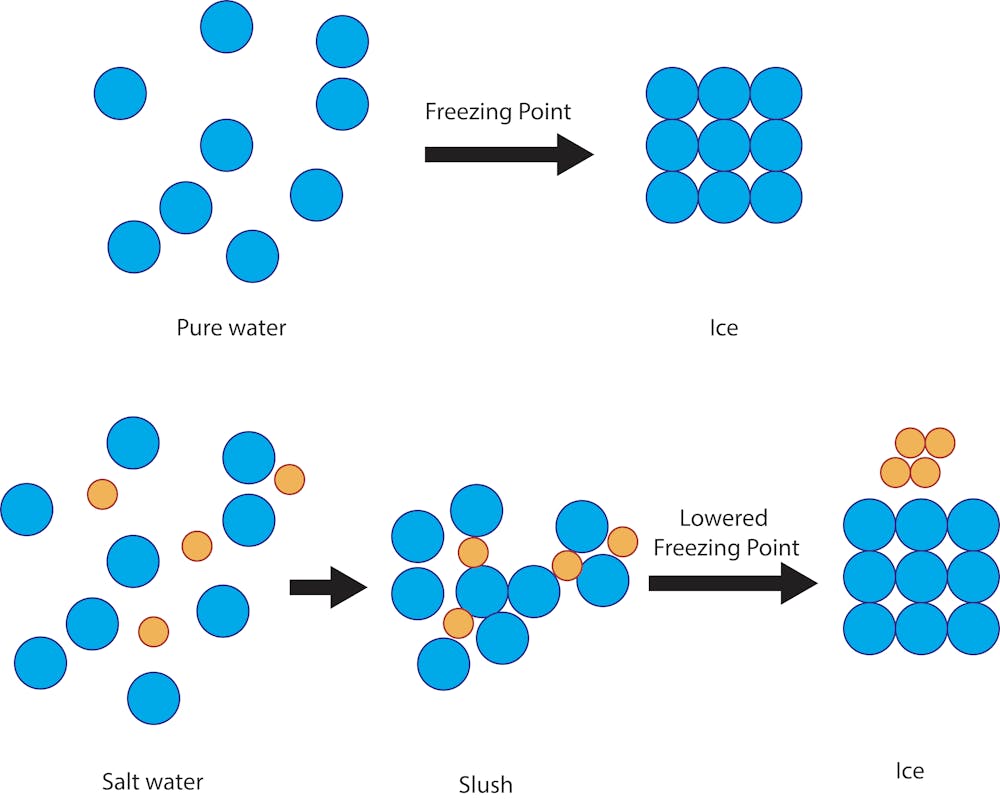
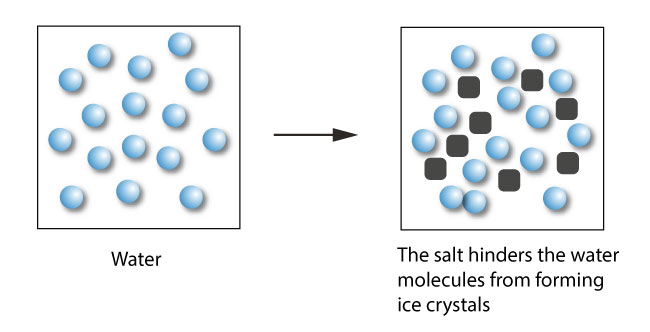
How to lower the freezing point of water. These ice crystals act as nucleation sites for other ice crystals to form. Any lower than that, and water must freeze. The greater the solute particles there are in a solution, the greater the decrease in freezing temperature.
One of these is by. A solution will have a lower freezing point than a pure solvent. Here ' s an example:
Overnight freeze water in multiple different containers. Salt lowers the freezing point of water because it forms ice crystals. The action of putting salt on ice lowers the freezing point, which means that the ice will melt at lower temperatures.
The first exception is when the solute stops dissolving. Instead, the salt will turn it into 0°f water. Oil won't suppress the freeze.
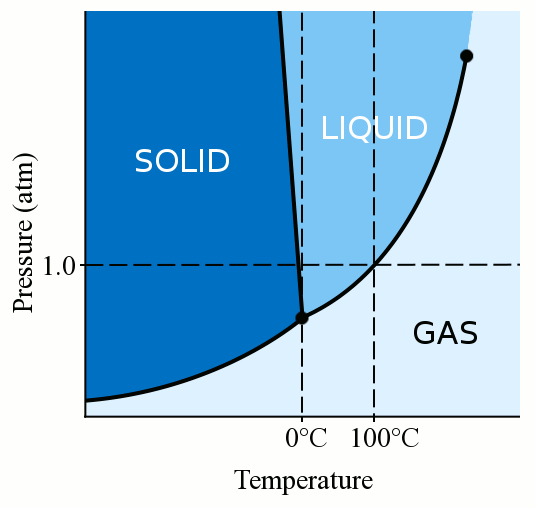
This process continues until the. How solutes affect freezing and boiling points? Ice forms when the temperature of water reaches 32.
What is the best substance to use to lower the freezing point of water? This is called the eutectic. While it's rare for temperatures to be that low in most places, you can raise your water's.

/the-freezing-point-of-water-609418_FINAL-01f50f5f4f7d4a39854bebcc59df1aa4.gif)
/the-freezing-point-of-water-609418_FINAL-01f50f5f4f7d4a39854bebcc59df1aa4.gif)








/the-freezing-point-of-water-609418_FINAL-01f50f5f4f7d4a39854bebcc59df1aa4.gif)